Yippee, it's a new year and once again, I say happy new year to you. Last year has been filled with alot of uncertainties, regardless, we thrive. In a bid to find possible solution and to contribute meaningfully to the research on going, aimed at fighting against cancer, I was involved in some research studies last year 2021, one of which was the main reason for absence for a very long time on social media in general.
I wish to share some of our findings from the research and also briefly discuss some key discoveries in the research work. This will obviously be the first time I am sharing a research work I am involved in. We basically worked on the role of Role of PKC in CD47-Mediated Phosphatidylserine Expression Pathway in Jurkat T Cells. Well, if you are not really into cell biology, this concept might be a little bit strange to you. Research findings are mostly meaningful when they are accessible by all.
Discovering something without making it accessible and known to the general public, of what use is the research? At times you might even have a lot to discuss on the subject matter but when you consider finance and the wordings (the more it is, the more you pay the publisher), you just have to cut some things off. Stemsocial is just a perfect place to pour out those thoughts without restraint. It won't be long before Stemsocial becomes a repository and bank of Information.
In the course of this article, I will be taking some excerpts from the original article and dropping some as quotes here and in the process giving more detailed explanation so that you won't be thrown off balance even when it's about becoming more confusing.
As usual, I will try as much as possible to break down facts were necessary so that you can atleast have something to take home. For sure, it will sound abstract as long as you are not into medical or biology related discipline, but worry less, that's why the Stemsocial is here : passing knowledge in another dimension with less ambiguity. I will start with the abstract of the work so that you can get a glimpse of the findings.
Abstract
Treatment failure in T-cell acute lymphoblastic leukaemia (T-ALL) occurs when leukemic blasts acquire resistance to chemotherapeutic agents. Current research efforts are focused on the search for targets for the development of more effective and less toxic anti leukemic drugs. CD47 has been suggested to be involved in chemo resistance and cell metastasis. Although several potential mechanisms were suggested to explain the therapeutic effect of CD47-targeting; the downstream effectors which lead to different effects by CD47 are still not well understood. In this preliminary study we assessed the role of Protein kinase C (PKC) in CD47-mediated phosphatidylserine expression pathway in jurkat T cells. Jurkat T cells were incubated with anti-CD47 mAb (BRIC 126), anti-CD44 mAb (BRIC 235) or control in the presence or absence of Bisindolylmaleimide I, hydrochloride (PKC inhibitor). Cells were stained with annexin-V FITC. Flow cytometry analysis was used for measurement of fluorescence intensity. Cell viability was detected using trypan blue exclusion test. PKC inhibition enhanced phosphatidylserine expression in CD47 receptor mediated leukaemia cells apoptotic pathway. This indicates that PKC may be involved in CD47- mediated PS exposure pathway in jurkat T cells. The observations from this study identify CD47 and PKC as novel functional proteins in jurkat T cells with promising therapeutic potential. This study would provide insight for targeted therapy against T- ALL disease
The major aim of our research work was was to assess the role of PKC in CD47-mediated PS exposure pathway in jurkat T cells.
We hypothesized that PKC may be involved in CD47-mediated PS externalization in jurkat. Our finding may help in the development of clinical strategies using PKC and CD47 as targets to further develop novel therapy for T cell leukaemia in humans.
Some terms I find ambiguous are T-cell acute lymphoblastic leukaemia (T-ALL), jurkat T cells, CD47 cells, PKC, Protein kinase C (PKC), and PS. Defining these terms and their respective roles in the subject of discussion before going deeper I to our findings will make our discussion much more interesting and easy to understand. Let's start primarily with leukaemia, a type of cancer.
Leukaemia by definition simply means cancer of the blood and associated with an outrageous increase in the number of white blood cells (cells that play significant role in immunity and protection against infections) as a result of their uncontrolled proliferation (increase in number) and rapid production. This is one unique attributes of cancer cells. When leukaemia is classified, it is mainly based on the type of white blood cells it affects and also based on how rapid the disease progresses.
Based on the type of white blood cells affected, it is mainly two namely - Lymphocytic leukaemia, this is also referred to as lymphoid or lymphoblastic leukemia. From the name, you can easily decipher that it mainly affect a type of white blood cells in the bone marrow known as the lymphocytes. On the other hand, the second is - Myeloid leukemia, it is also referred to as myelogenous leukemia which has the potential of starting from other blood cells (like the platelets and red blood cells) other than than lymphocytes.
Now based on how fast and rapid the cancer cells proliferate and gets worsened, it could either be Acute leukaemia (the fast growing type) or chronic leukemia (slow growing type).
In acute leukemia, the cancer cells are usually very fast thus leading to overwhelming produced number of white blood cells that are immature and practically has no role, more or less inactive. These immature cells with time dominate the blood system also overshadowing the good cells inside the bone marrow. This results to a decline in the function of the bone marrow, whose job is to produce blood cells that are healthy and functional.
When it comes to chronic leukaemia, they are usually slow in growth. More like "slow poisons" but still very dangerous. Their gradual progression leads to the accumulation of mature yet but abnormal and useless cells. They are mostly noticed when the condition has worsened through the symptoms associated with them and this is one of the reasons they are very difficult to treat. Having said all the above, we can confidently summarize the types of leukemia into 4 namely:
•Acute lymphocytic leukemia •Acute myeloid leukemia •Chronic myeloid leukemia and •Chronic lymphocytic leukemia
With the above explanation, you can noe see where our research topic is headed to, which is Acute lymphocytic leukaemia. T cells or lymphocytes are part of the human immune system as explained earlier and they also play a role in fighting cancer. A decline in this activity is one of the factor account for the invasiness of cancer. Now that we have a good understanding of leukemia and its classification, lets explain other terms and discuss our findings.
The etiology of Acute Lymphocytic Leukemia is unknown. However, certain environmental factors have been implicated in the etiology of Acute Lymphocytic Leukemia such as exposure to benzene, ionizing radiation, or previous exposure to chemotherapy or radiotherapy.
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer although it affects adults as well but more in children. I will be limiting the discussion to only ALL, since it is the subject of focus.
You may not or may not be familiar with these type of cell known as the CD47. Reading this will sure add an extra knowledge. CD47 is a 47-52kDa transmembrane glycoprotein of the immunoglobulin (Ig) super-family. These cells is highly glycosylated (i.e it is has units of glucose residues attached to it) and it is expressed by all the cells in the body system and without question, expressed well in the lymphocytes. The presence of this cell on leukaemic stem cells is one of the reasons why the leukaemic cancer cells evade the destructive effects of the body immune defense mechanism.
These CD47 cells block and inhibit the phagocytic action of phagocytes (group of cells that protect the body by ingesting harmful foreign particles) like the neutrophils, monocytes, macrophages, mast cells, and dendritic cells. CD47 mechanism of action is quite simple; through a regulatory molecule called alpha, SIRPα, they bind to the phagocytes and initiate a process known as signal transduction cascade. The cascade of reaction leads to the inhibition of the phagocytes and thus rendering them powerless against the leukaemia cells. As a result of this, the cancer cells go unchecked and unharmed by the host immune system. So in short, CD47 expression on leukaemic cells is anti-phagocytic in nature.
CD47 activation induces apoptosis of B-cell chronic lymphocytic leukaemia cells through a caspase-independent mechanism. Recently, CD47 was reported to be a marker of tumour-initiating cells in leukaemia and bladder cancer. The ligation of CD47 with monoclonal antibodies, thrombospondin-1 (TSP-1) or the specific CD47-binding peptide 4N1K, derived from the C- terminal binding domain of TSP-1, induces phosphatidylserine exposure as part of apoptosis in blood cells.
While we keep the above important fact in mind, let's bring in another molecule that is highly indicated in the phagocytosis - Phosphatidylserine. This molecule has also been indicated to have a role to play in all these.l, through it's interaction with an enzyme called protein kinase C.
Phosphatidylserine (PS) by nature is a phospholipid and it is one of the component of the cell membrane that has a role to play in cell replication cycle as regards apoptosis (a natural pathway through which cells die off). Some pathogens like viruses have the ability of mimicking this pathway thus, gaining entrance into their host undetected.
Very importantly, whenever this molecule Phosphatidylserine is exposed or expressed on the surface membrane of any cells, it marks the cell for ultimate destruction through the process of apoptosis. What this means is that, you will mostly find them on naturally aging cells in the body. Aged cells die off through apoptosis but in cases of pathogen inflicted diseases, the mechanism of death is through necrosis
Lets briefly talk about Protein kinase C (PKC), an enzyme also implicated by its interaction with CD47. PKC is a family of kinases comprising at least 11 subspecies and it majorly involved in the regulation of most protein functions. They are ubiquitous and can be found virtually in all cells.
CD47 and protein kinase C (PKC) have been reported to interact with these enzymes and with other chemical molecules leading to the exposure of PS on the outer leaflet thereby regulating different cellular functions. The expression of PS on outer cell membrane is instrumental in triggering blood clotting and also serves as an "eat me" signal for the clearance of apoptotic cells.
The isoforms of PKC (forms of PKC that are similar in function but differ by structural composition) are differentially localised by their association with anchoring proteins called RACK (receptors for activated C kinase) or PICK (Proteins that interact with C kinase).
Report have shown that, the direct binding of CD47 by the molecule called thrombospondin-1, TSP-1, a natural inhibitor of tumorigenesis in healthy tissue.) readily inhibits soluble guanylcyclase (an enzyme that drives activation of transduction mechanism in cells) in jurkat cells, and the inhibition requires an increase in cytoplasmic Ca2+ levels. I am not surprised at the involvement of Calcium because it is natural activator of many cascade mechanisms.
Our discourse won't make any sense without finally talking about the the main cell used in the research study - Jurkat cells.
Jurkat cells are an immortalized line of human T lymphocyte cells used to study acute T cell leukaemia, T cell signalling, and the expression of various chemokine receptors.
These cells were first originally gotten from the peripheral blood of a boy with T cell leukemia and one of their main uses is to study study most cellular events in T cell biology, such as T cell signaling and molecular events in the HIV infection life cycle. They are one of the cells that are indispensable when one wants to study and determine the mechanism of differential susceptibility of most cancer cells as relating to radiation and drugs.
Evidences have shown that there are some protein kinase C PKC activities in these jurkat cells. Recall that CD47 is anti-phagocytic while PS expression is like a signal for apoptosis. Besides, PKC function in CD47-PS exposure pathway has not been investigated in jurkat cells and because of this gap in knowledge, this research work was birthed.
Findings and discussion
All the Jurkat cells incubated in HBSS medium alone (control) for 24 hours expressed a low level of PS (mean % annexin V positive cells 3.133 ± 0.186, n =3, Fig. 1A). This indicated that the medium did not induce any significant increase in PS expression during the culture period. The addition of BRIC 126 was shown to induce an increase in the level of PS expression on Jurkats cells (Fig. 1A).
In contrast, BRIC 235 (mean % annexin V positive cells of BRIC 235 (3.000 ± 0.116, n=3) was not shown to induce any significant increase in PS expression, compared with the control (p=0.5748). BRIC 126 (mean % annexin V positive cells, 9.267 ± 1.462, n=3) induced a significant increase in PS expression in comparison with BRIC 235 (p=0.0129). BRIC 126 also induced a significant increase in PS expression when compared with control (p= 0.0141) (Fig. 1A).
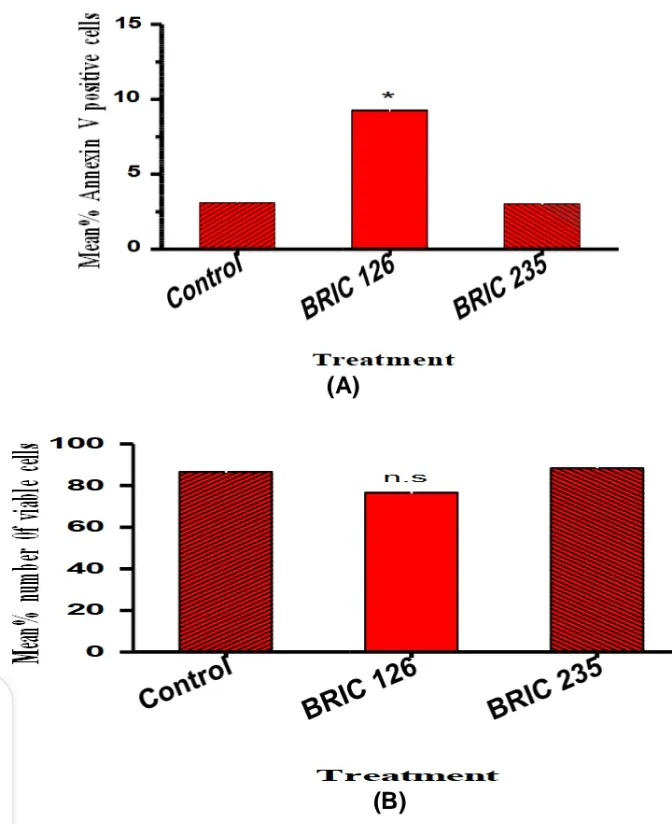 Figure 1: Phosphatidylserine exposure and apoptosis; (A) Histogram showing % annexin V positive cells following treatment with BRIC 126, BRIC 235 or control. The preliminary data shows that BRIC 126 significantly induced more PS when compare with BRIC 235 or control (P<0.05). The data are presented as means ± SD of the percentage of annexin V-positive cells of individual treatments from three different passages. (B) Histogram showing means % number of viable cells following treatment with BRIC 126, BRIC 235 or control. The preliminary data shows that BRIC 126 had more dead cells when compared with BRIC 235 or control (p>0.05). The data are presented as means ± SD of the % viable cell counts of individual counts from three different passages. Statistical analyses were performed using the Student’s t-test (*) indicates significance for p<0.05; n.s- non-significance for p>0.05) Percentage viable cell count showed that cells treated with BRIC 126 showed no significant decrease in mean % viable count when compared with BRIC 235 (76.67 ± 8.819 vs. 88.33 ± 1.667, n=3, p=0.3486) or Control (76.67 ± 8.819 vs. 86.67 ± 3.333, n=3, p=0.2635). It is noteworthy that PS showed an association with a concomitant loss of Jurkats viability in vitro (Fig. 1B).
Figure 1: Phosphatidylserine exposure and apoptosis; (A) Histogram showing % annexin V positive cells following treatment with BRIC 126, BRIC 235 or control. The preliminary data shows that BRIC 126 significantly induced more PS when compare with BRIC 235 or control (P<0.05). The data are presented as means ± SD of the percentage of annexin V-positive cells of individual treatments from three different passages. (B) Histogram showing means % number of viable cells following treatment with BRIC 126, BRIC 235 or control. The preliminary data shows that BRIC 126 had more dead cells when compared with BRIC 235 or control (p>0.05). The data are presented as means ± SD of the % viable cell counts of individual counts from three different passages. Statistical analyses were performed using the Student’s t-test (*) indicates significance for p<0.05; n.s- non-significance for p>0.05) Percentage viable cell count showed that cells treated with BRIC 126 showed no significant decrease in mean % viable count when compared with BRIC 235 (76.67 ± 8.819 vs. 88.33 ± 1.667, n=3, p=0.3486) or Control (76.67 ± 8.819 vs. 86.67 ± 3.333, n=3, p=0.2635). It is noteworthy that PS showed an association with a concomitant loss of Jurkats viability in vitro (Fig. 1B).
In order to demonstrate the role of PKC in CD47-mediated PS exposure pathway in jurkat cells, the experiment was repeated in the presence of 1.5 μM of Bisindolylmaleimide I, hydrochloride. It was observed that (BRIC 126 + Bis I) induced a significant higher PS exposure than BRIC 126 alone (11.933 ± 0.636 vs. 9.267 ± 1.462, n=3, p=0.03, Fig. 2A and 3).
(Control + Bis I) did not show any significant difference in PS expression when compared with Control (4.667 ± 0.882 vs. 3.133 ± 0.186 n=3, p=1.1641). In addition (BRIC 235 + Bis I) did not show any significant difference in PS when compared with BRIC 235 alone (3.667 ± 0.882 vs. 3.000 ± 0.116, n=3, p=0.4952, Fig. 2A and 3). Percentage viable cell count showed that cells treated in the presence of Bis I all had lower mean % viable cells than cells treated without the PKC inhibitor.
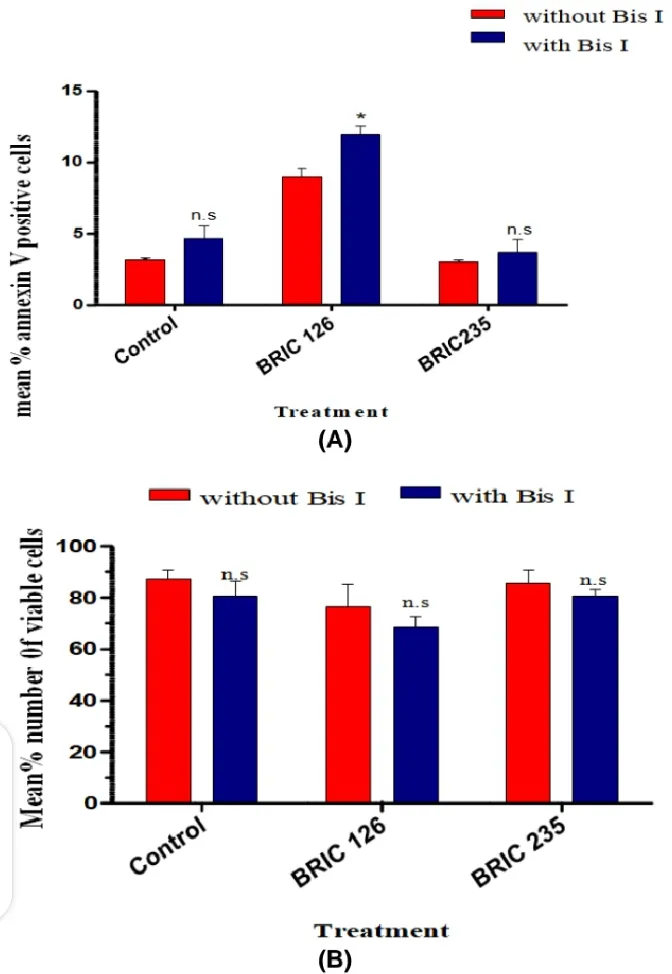
Fig. 2. Phosphatidylserine exposure and apoptosis after PKC inhibition; (A) Histogram showing % annexin V + cells following treatment with BRIC 126, BRIC 235 or control in the presence or absence of Bisindolylmaleimide I, hydrochloride (Bis I). Note that cells treatment with BRIC 126 in the presence of Bis I exposed more PS when compared with BRIC 126 alone. The data are presented as means ± SD of the percentage of annexin V+ cells of individual treatments from three independent experiments. (B) Histogram showing mean % number of viable cells following treatment with BRIC 126, BRIC 235 or control in the presence or absence of Bis I. Note that treatment with BRIC 126, BRIC 235 or control in the presence of Bis I produced higher amount of dead (non-viable) cells when compared with respective treatments alone. The data are presented as means ± SD of the % viable cell counts of individual counts from three different passages. Statistical analyses were performed using the Student’s t-test with p<0.05 (*).
For (BRIC 126 + Bis I) vs. BRIC 126 (68.33 ± 4.410 vs. 76.67 ± 8.819, n=3, p=0.4683); (BRIC 235 + Bis I) vs. BRIC 235 (80.33 ± 5.175 vs. 88.33 ± 1.667, n=3, p=0.4469); and Control + Bis I vs. Control (80.67 ± 5.783 vs. 86.67 ± 3.333, n=3, p=0.3696). It is noteworthy that PS exposure in the presence of PKC inhibition showed an association with a concomitant but non significant loss of Jurkats viability in vitro (Fig. 2B) above.
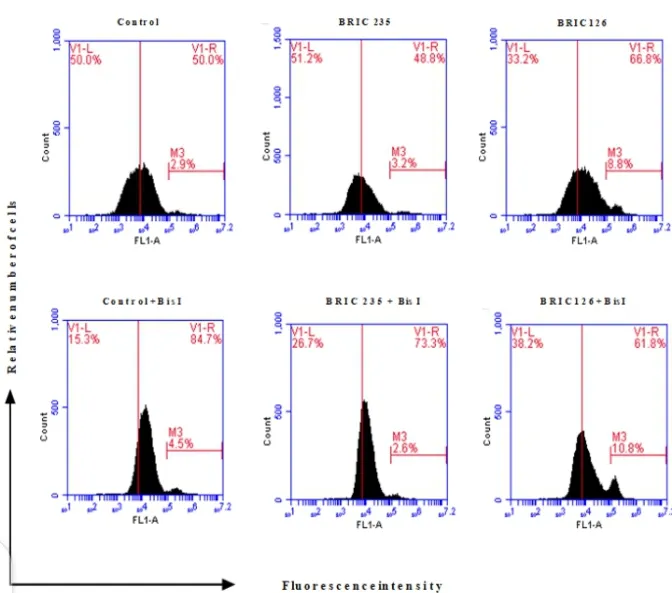
Figure 3: Flow cytometer profile showing PS exposure in jurkat cells; Note the % shift (M3) in the histogram for the different treatments; most especially between BRIC 126 and Control or (BRIC 126 + Bis I). The filled-black histograms show the annexin V positive cells (PS levels) resulting from each treatment, M3 is marker showing % shift and quantifies annexin V positive cells; V1-L indicates % space left to the vertical marker, V1-R indicates % space right to the vertical marker; FL1-A means the fluorescence intensity of Annexin V FIT-C
CD47 ligation with anti-CD47 mAb (BRIC 126) caused the exposure of PS, which is consistent with previous studies. Cells treated with BRIC126 showed a higher PS exposure than BRIC 235 or control. This demonstrates that CD47 molecules are expressed on the cell membrane of jurkat cells and also supports previous study that ligation of CD47 with anti-CD47 mAb induce PS exposure, hence apoptosis in these cells.
Treating ALL usually involves 3 phases namely - the induction (remission induction), consolidation and lastly maintenance phase. Generally, treatment of cancer of any kinds is never without any side effects and one of which is harm to healthy cells. Chemotherapy is one way and for the treatment of ALL, it can take up to about 2 years or more depending on the severity.
PKC δ (an isomer of PKC) induces DNA oxidation and ROS (Reactive oxygen species) overproduction leading to apoptosis of L- buthionine-S, R-sulfoximine resistant neuroblastoma cancer cells and potentiates the cytotoxic effects induced by L-buthionineS,R-sulfoximine in sensitive cells.
Treating ALL usually involves 3 phases namely - the induction (remission induction), consolidation and lastly maintenance phase. Generally, treatment of cancer of any kinds is never without any side effects and one of which is harm to healthy cells. Chemotherapy is one way and for the treatment of ALL, it can take up to about 2 years or more depending on the severity.
PKC δ (an isomer of PKC) induces DNA oxidation and ROS (Reactive oxygen species) overproduction leading to apoptosis of L- buthionine-S, R-sulfoximine resistant neuroblastoma cancer cells and potentiates the cytotoxic effects induced by L-buthionineS,R-sulfoximine in sensitive cells.
Results from this experiment demonstrated the involvement of PKC in CD47-PS exposure pathway in jurkat cells. This is worthy of note for future work. Therefore, further research where specific inhibitors of individual PKC isoforms are included in the assay, would be helpful to unravel the role of PKC isoforms in this novel pathway in jurkat cells; using maybe human peripheral blood T cells as control cells.
Treatment with targeted drugs such as imatinib are usually used for treatment. In the last phase of treatment - the consolidation phase, this is more like a stabilizing stage where the chemo shots are now given gradually over a period of time.
During the induction stage, it is mainly aimed at sending the cancer cells into remission (complete remission). Remission is a stage where by the cancer cells of the white blood cells are no longer found in the bone marrow sample of the patient. At this point, everything returns to normal and the patient is able to carry on with their normal life. The cancer cells being in remission doesn't necessarily mean it has been cured. It can be in remission for years, so patients only will have to once in a while be examined to know the stage of the cancer before another chemotherapy. ALL is not as serious as CML (Chronic Myeloid leukemia).
Research is aimed at finding lasting cure to cancer disease which has taken more life than we can think of. Until the lasting cure is found, we continue to research for novel ideas, pathways that can be altered to permanently inhibit cancer cells.
If you didn't get anything from this article, just remember that ALL is treatable and that PKC pathway can be inhibited, thereby ultimately halting cancer cells progression. Having ALL is not a death sentence.
For full article request and to see in detail, the materials and methodology as well as the full discussion, kindly inbox me on discord @cyprianj. Terms and conditions apply.
Ps: All images with exception of the first one are owned by us "Research team" and cannot be shared without due consent.
It can also be accessed as a book chapter in the Journal - Current Aspects in Pharmaceutical Research and Development Vol. 2, Chapter: 10, Publisher: B P International.
DOI: https://doi.org/10.9734/bpi/caprd/v2/13381D
Thanks for reading.... Happy new year once again.
References •Jurkat Cell Line •Typical Treatment of Acute Lymphocytic Leukemia (ALL) •Protein Kinase C •Types of leukemia •Acute Lymphocytic Leukemia •Acute Lymphoblastic Leukemia
Return from Original Research: Discussion on the role of Protein Kinase C in Acute lymphoblastic leukaemia to cyprianj's Web3 Blog