The proposed automatic molecular reconfiguration and remodelling of the lead molecule using specialised computer model might make things much more easier. This is a huge advancement in the science of drug development. Before I delve into how this automated model works, let me quickly give you a heads up about drug discovery and development probably you'll get to understand what necessitated the discovery.
What if I tell you that it takes not less that ten years (10 years) for a newly discovered drug to reach the market. Drug biosynthesis is very costly, complex, tedious, competitive, highly risky and undergoes a lot of checks before general use and acceptance.
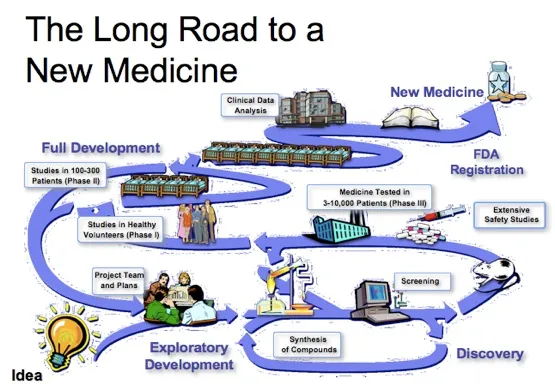
The process of bringing new pharmaceutical drugs to the market once a lead molecule has been identified through the process of drug discovery is known as drug development. Drug discovery begins with the identification of a lead molecule with desired properties. Please don't get confused, the lead molecule here refers to that particular chemical substances that has a desired therapeutic potency which interest the chemist.
Extraction of the active molecule needed
During the process of drug development, the first stage is the synthesis/isolation of the desired lead molecule from the parent compound and this process takes about one to two years.
The extraction of the lead molecule with desired properties can be one of the most challenging task for the chemist and most times it is done manually from natural sources, chemical sources, rational approach, molecular modelling, combination chemistry or through biotechnology (Recombinant DNA technology). Once the lead molecule is extracted, the real deal now begins.
Now that the lead molecule has been isolated from the parent compound, what next? 🤔
It has to be tested on animals first
The preclinical studies which is the stage 2 of preclinical studies involve a whole lot of tests using animals that share similar physiologic system with humans. At this stage the drug is tested on smaller animals like mice guinea pigs, rabbits before proceeding to test it on bigger animals like dogs, pigs etc. so to expose the whole pharmacological profile of the drug. If you haven't heard, whatever will kill a Guinea pig will definitely kill a man. This is the reason this animal is mostly used for experimental studies especially in pharmaceuticals.
The preclinical studies takes about two to four years (2-4 years) to be completed. At the preclinical stage, the Lethal Dose 50, LD50 (the concentration of a drug that will kill half the population of animals of interest). Every phase is a check point and if it is discovered that the drug is likely to be toxic and cause serious threat, the whole process is stopped.
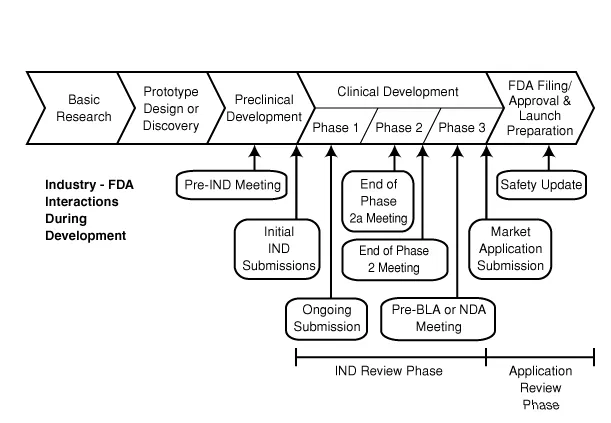
License to conduct clinical trials using humans
The whole findings and acclaimed therapeutic efficacy of the drug are documented and passed to the regulatory agency for grant of Investigational new drug licence. This is the third stage and takes about another 3-6 months or even a full year. If the license is approved then the pharmaceutical company proceeds to conduct clinical trials with the drug using humans. In addition to the above, the pharmaceutical formulation and dosage of the drug is also set at this stage. Always remember that
without dosage, the administration of drugs can seemingly be likened to overdose and abuse. So it becomes pertinent that the dosage of the drug be set before administration during clinical trials.
The clinical trials begins
The clinical trials which can last for about 6-10 years has phase I-IVand it is the various phases a proposed drug has to pass before it is fully commercialised. During the phase I, the human safety, pharmacological properties (the effect of the drug on the body) and pharmacokinetic properties (how the body deals with the drug in terms of absorption, distribution, metabolism and excretion) are checked.
The number of patients used for the trial are usually very small and emphasis is based mainly on tolerability and safety of the drug. After phase I, it goes through the phase II where its therapeutic effect is explored as well as the dose range at which the drug will be more effective. The therapeutic index (the ratio of the largest amount or dose of a drug producing no toxic effect to the amount that will provide a cure) is also checked. We are dealing with humans here and as such extreme measures are taken to stories that touch the heart.
In Phase III, the acclaimed therapeutic efficacy of the drug is tested and confirmed. Peradventure the drug works for its sole purpose, a New drug Application form is submitted to the regulatory agency for approval and license to market the drug. This phase III is done in larger settings like teaching hospitals and federal government hospitals and the number of patients used are very large.
In the last phase, phase IV is for post marketing surveillance. The drug has been approved and it's already in market but still under observation for any future reaction. In a situation where the drug produces unwanted side effects that can could be fatal fatality cause disease mutation or cancer, it is recalled from the market and it's further production halted.
The thalidomide disaster in Germany
Take for instance the case of thalidomide disaster, thalidomide was a drug used by pregnant women in Germany to reduce pain associated with labour, but it turned out that this drug had teratogenic effect (substances or drug that have the ability to cause congenital malformation) and this resulted to children born with several congenital malformations of the limbs and a typical example is phocomelia (children born with missing bone and flipper- like hands or feet). This drug was later banned from usage.
Do we also talk about the case of a teething agent called 'my pikin' which killed many children in Nigeria, the case of the popularly used 'ajinomoto' - a seasoning agent used in cooking which was found to contain substances that could be carcinogenic (substances that can cause cancer) or the use of 'bromate' in bakery. Most of these were found to have negative effect and were later banned from use by the regulatory agency- National agency for food drug administration and control (NAFDAC) in Nigeria.
When does the drug development stop?
Drugs continue their development even after going into the market and uncommon adverse effects that occurs after prolonged usage are detected at the phase IV. Though most times contraindications are stated and most importantly for pregnant women. Some drugs could have life long damage to organs e.g Gentamycin, an antibiotics which is notorious for its three known major effect which are nephrotoxic effect (damages the kidney), hepatotoxic effect (damages the liver) and ototoxic effect (damages the ear) in children is highly contraindicated in pregnancy because they have much of their effect during organogenesis (the process or stage of organ development in infants)
why is that teeth not white?
Other drugs like tetracycline and doxycycline are antibiotics known to discolor the developing teeth of children under the age of 8 years when administered. They give the teeth a yellow colour and this is most times permanent. This is one of the reason it's use is contraindicated in pregnancy. Now you know why some people's teeth are not white as it ought to be, though drugs are not the only substances that could give the teeth a shade of color. Mind the kind of drugs you give to children, or the kind of drug you take while pregnant. It is always advisable to see your doctor before administration of any drug. You might actually be causing more harm than good to that innocent child.
Now imagine the whole series of stages and phases required for drug development, I guess you can now see that the whole process is really hectic, time consuming and risky indeed. We all know that the sole aim of producing drugs is to make treatment faster and most drugs produced are for humans use thus, the need to highly regulate it.
But the big question here is this: Can't we have a shorter way of new drug discovery? 🤔Can't we explore other ways of making drugs easily synthesised and made available for human use? Must we have to pass through the rigorous step? 🙄I think the MIT researchers might have some answers to give us today.
I present to you👇
Automated drug molecule model design : a likely way out
Technology has been so dynamic that it has cut across virtually all spheres of human life. Name any discipline and you are definitely going to find the application of technology in one or two aspects. The production of drugs have gone far way beyond the use of only manual methods. Automation in medicine through biomedical engineering has not only made therapeutics less burdensome but has made it much more effective.
The whole essence of remodification
Like I explained earlier, when the lead molecule has been isolated, further work is done on this molecule manually. The molecule might not really have all the desired properties the chemist wants. In order to achieve his desired goal, he begins a lot of modifications on the drug and this involves addition and removal of function groups (a group in a molecule that basically determines the chemical property and the reaction), alteration in the group property to either make it more hydrophilic (water soluble or polar) ) or hydrophobic (non water soluble or non polar). He continues to add, remove break bonds untill he gets the perfect lead molecule.
Any guarantee of success after remodification?
The whole process of remodification and reconfiguration of the functional groups can take a long time to complete and even after the whole process, there is still no certainty that the desired quality or characteristics will be gotten. What this invariably means is that the chemist only succeeded in wasting his time and it is even possible that there could be a mistake or human error while carrying out remodification.
Here comes computer automated model for drug molecule design
MIT researchers have recently developed a model that can select a new lead molecule for drug development, the manual method will in no time become obsolete should the prototype be put into clinical use. These researchers developed a computer model that could automatically modify a lead molecule until a desired one with therapeutic efficacy and potency is gotten. This automated model selects the best lead molecule with good properties, modifies it and at the same time ensures that the molecule is still chemically valid.
How does this automated modelling really works?
I think it is time you hear from the horse's mouth
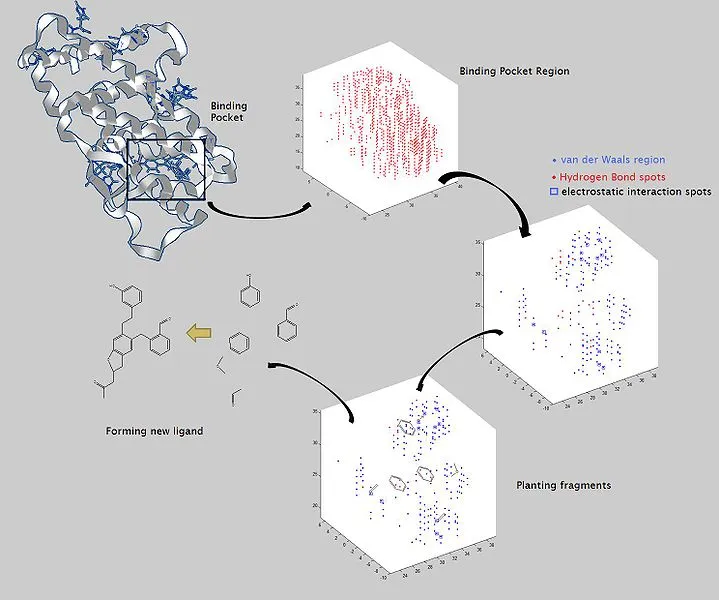
The model basically takes as input molecular structure data and directly creates molecular graphs — detailed representations of a molecular structure, with nodes representing atoms and edges representing bonds. It breaks those graphs down into smaller clusters of valid functional groups that it uses as “building blocks” that help it more accurately reconstruct and better modify moleculesMIT
It doesn't stop there, after remodification another question you might want to ask is: does the new molecule formed conforms to chemical laws? Yes it may be valid but does it fit into the principles backing chemical substances. Automated model of drug design is mainly faced with the problem of validity and conformation of the new molecule formed to chemical laws. Since the model runs on the generation of the molecular graph of the lead molecule, producing new molecule with better desired qualities will be faster and less tedious.
The picture above is a typical example of a lead molecule undergoing automated molecular remodification. The bonds and functional groups are broken at first instance only to be rearranged later to produce a more potent and valid molecule that conforms to all chemical rules.
The manual method which involves try and error will likely be a thing of the past. All the model needs is the input data of the molecular structure of the lead molecule in question and in no times produces a better molecule with desired properties. This model will speed up drug isolation and development. We might have a shorter route to development of new medicine provided this is clinically applied.
The machine learning model
Another fascinating area is that of machine learning model. This model will definitely put smile the faces of pharmaceutical industries as they may not have to worry much about the rigorous process planning chemical synthesis pathways. The machine model selects best pathways that could actually give the best desirable product.
Machine learning can help plan chemical synthesis pathways and help identify which chemical parts within a molecule contribute to particular properties, this may ultimately lead us to explore new chemical spaces, increase chemical diversity, and give us a larger opportunity to identify suitable compounds that will have specific biological functions. The application of machine learning tools provides an opportunity to augment and accelerate drug discovery and development — and get new medicines to patients more quickly, MIT
Conclusion
The long journey and conventional paradigm of drug discovery and development maybe subject to adjustment so as to suit the fast advancement in the area of science and technology in medicine. The driving factor for improvement in drug development is to provide effective, efficient and prompt adequate health care services to the patient.
The more faster and effective drug development is, the more and quicker means through which diseases are eradicated. But wait, in as much as we are trying to find means of making drug development faster, the safety of humans life is also a critical issue worth putting into consideration and it involves no rushing, rather a systematic and gradual approach. It only gets better as we improve in science and technology and in the same time putting safety of man as number one priority.
Until next time😉✌️
MIT images are free to use provided you credit the MIT researcher if the picture bears the author and if otherwise, credit is given to the MIT researchers as a whole. A detailed explanation of this can be found here in the comment section of the article. You can also read up MIT terms for use of images in the first and second reference below
References
¶automating molecule design ¶applying machine learning model to challenges in pharmaceutical industry ¶Thalidomide ¶teeth discolouration ¶drug discovery and development ¶Role of bioinformatics and pharmacogenomics in drug discovery and development process
If you write STEM Science Technology Engineering Mathematics related posts, consider joining @Steemstem and @Stemng
Return from The Advent of automated molecular reconfiguration and likely remodification in the paradigm of drug discovery and development to cyprianj's Web3 Blog